Gas sensors
Gas detectors can be used to detect combustible, flammable, and toxic gases and oxygen depletion. Modern society frequently encounters the use of both toxic and flammable gases on a daily basis. Gases are utilized in a controlled manner in a wide range of applications however it is essential that we employ critical safety systems to ensure we regulate and control the potential risks associated with an uncontrolled gas leak.
Four basic types of gas sensors are widely used for gas detection and monitoring in commercial or industries:
Catalytic gas sensors:
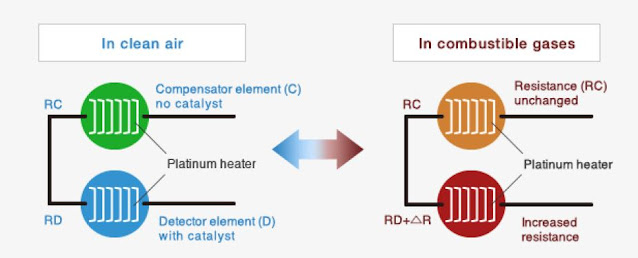
The catalytic-type gas sensor consists of two elements:
- A detector element (D): which contains catalytic material and is sensitive to combustible gases, and
- A compensator element (C): which is inert.
Normally, a Wheatstone bridge circuit is formed with both elements as shown in Figure 2. A variable resistor (VR) is adjusted to maintain a state of balance of the bridge circuit in clean air free of combustible gases.
Figure 2: Basic Measuring Circuit
When combustible gases are present, only the resistance of the detector element will rise, causing an imbalance in the bridge circuit, thus producing an output voltage signal (Vout). The output voltage signal is proportional to the concentration of combustible gases as shown in Figure 3. Gas concentration can be determined by measuring the output voltage.
Figure 3: Relationship between gas concentration and output voltage for catalytic gas sensor
Electrochemical gas sensors
Figure 4: Operating principle of the electrochemical gas sensor
Electrochemical-type gas sensors are amperometric fuel cells with two electrodes. The basic components of two electrode gas sensors are a working (sensing) electrode, a counter electrode, and an ion conductor in between them. When toxic gas such as carbon monoxide (CO) comes in contact with the working electrode, oxidation of CO gas will occur on the working electrode through a chemical reaction with water molecules in the air (see Equation 1).
CO + H2O → CO2+ 2H+ + 2e- …(1)
Connecting the working electrode and the counter electrode through a short circuit will allow protons (H+) generated on the working electrode to flow toward the counter electrode through the ion conductor. In addition, generated electrons move to the counter electrode through the external wiring. A reaction with oxygen in the air will occur on the counter electrode (see Equation 2).
(1/2)O2 + 2H+ + 2e- → H2O …(2)
The overall reaction is shown in Equation 3. Figaro Electrochemical-type gas sensor operates as a battery with gas being the active material for this overall battery reaction.
CO + (1/2)O2 → CO2 …(3)
By measuring the current between the working electrode and the counter electrode, this electrochemical cell can be utilized as a gas sensor.
The theoretical equation for CO detection
In order to measure the sensor’s output current, it must be connected to an external circuit. By controlling gas flowing toward the working electrode with diffusion film, the output current flowing across the external circuit will be proportional to the gas concentration (see Equation 4 and the chart at the right).
Figure 5: Relationship between CO gas concentration and output current for the electrochemical gas sensorThe linear relationship of gas concentration to sensor output makes this technology ideal for gas sensing applications.
I = F × (A/σ) × D × C × n …(4)
where:
I: Sensor output
F: Faraday constant
A: Surface area of the diffusion film
σ:Thickness of the diffusion film
D: Gas diffusion coefficient
C: Gas concentration
n: Number of reaction electrons
Metal Oxide Semiconductor (MOS) gas sensors
When semiconductor particles (typically tin dioxide) are heated in air at high temperature, oxygen is adsorbed on the particle surface by capturing free electrons. The depletion layer thus formed is largely dependent on the radius of semiconductor particles used.
If it is as small as conventionally used in gas sensors (tens nano-meters), the depletion can extend up to the whole area of each particle (volume depletion, high sensitivity). If the size is far larger, on the other hand, depletion takes place conventionally on the periphery of each particle (regional depletion, low sensitivity).
Figure 6. Energy band structure (top) and distribution of conduction electrons (bottom) for a semiconductor particle as correlated with an increase in adsorbed oxygen concentration
Figure 6 shows how the energy band structure and the distribution of conduction electrons change with increasing the partial pressure of oxygen from zero (flat band state) to state I (regional depletion), II (border), and III (volume depletion). Until the border is reached, the adsorption equilibrium is attained by increasing the depletion layer thickness. Later (volume depletion), however, the Fermi level is lowered by p kT ongoing from II to III while the layer thickness is kept constant.
In this stage, two important equations are derived theoretically for a sensor device consisting of spherical particles, as follows.
[e]S=Nd exp{-(1/6)(a/LD)2-p} ... (1)
R/R0=Nd/[e]S ... (2)
Here [e]S is the surface electron concentration of particles and LD is the Debye length. R and R0 is the sensor resistances at the steady-state and flat band state, respectively. For other symbols, see the caption of Fig.6.
When sensor materials are selected, Nd, a, LD and R0 are fixed, while p is dependent on the actual gaseous conditions.
As described above, MOS type gas sensors change resistance (R) as a result of a change in adsorbed oxygen concentration. If this is used adequately, one can detect reducing gases like carbon monoxide. The adsorbed oxygen formed in clean air will be consumed on contact with carbon monoxide, the resulting decrease of R being used to estimate the concentration of carbon monoxide. The sensor recovers the original level of resistance when carbon monoxide is off. Such a detection mechanism is operative in tin dioxide-based gas sensors.
Summary of operation
STEP1
In clean air, donor electrons in tin dioxide are attracted to oxygen which is adsorbed on the surface of the sensing material, preventing electric current flow.
STEP2
In the presence of reducing gases, the surface density of adsorbed oxygen decreases as it reacts with the reducing gases. Electrons are then released into the tin dioxide, allowing current to flow freely through the sensor.
Figure 7: Basic principle of operation of MOS gas sensor
Infrared gas sensors:
Infrared gas detection is a method for detecting combustible hydrocarbon gas with infrared light. The detector consists of a source of infrared light, an optical filter to select the proper wavelength, and an optical infrared receiver.
As the gas flows into the space between the source and receiver, hydrocarbon molecules in the gas absorb some of the infrared energy. The receiver detects this drop in received energy as a measure of the amount of hydrocarbon gas present.
An Infrared Gas Detector often uses two wavelengths of infrared energy with one active wavelength used for gas absorption, and the other as a reference wavelength to compensate the output signal of the Infrared Gas Detection system for the effects of temperature, humidity, and the presence of moisture or dirt on the optical filters.











Comments
Post a Comment